
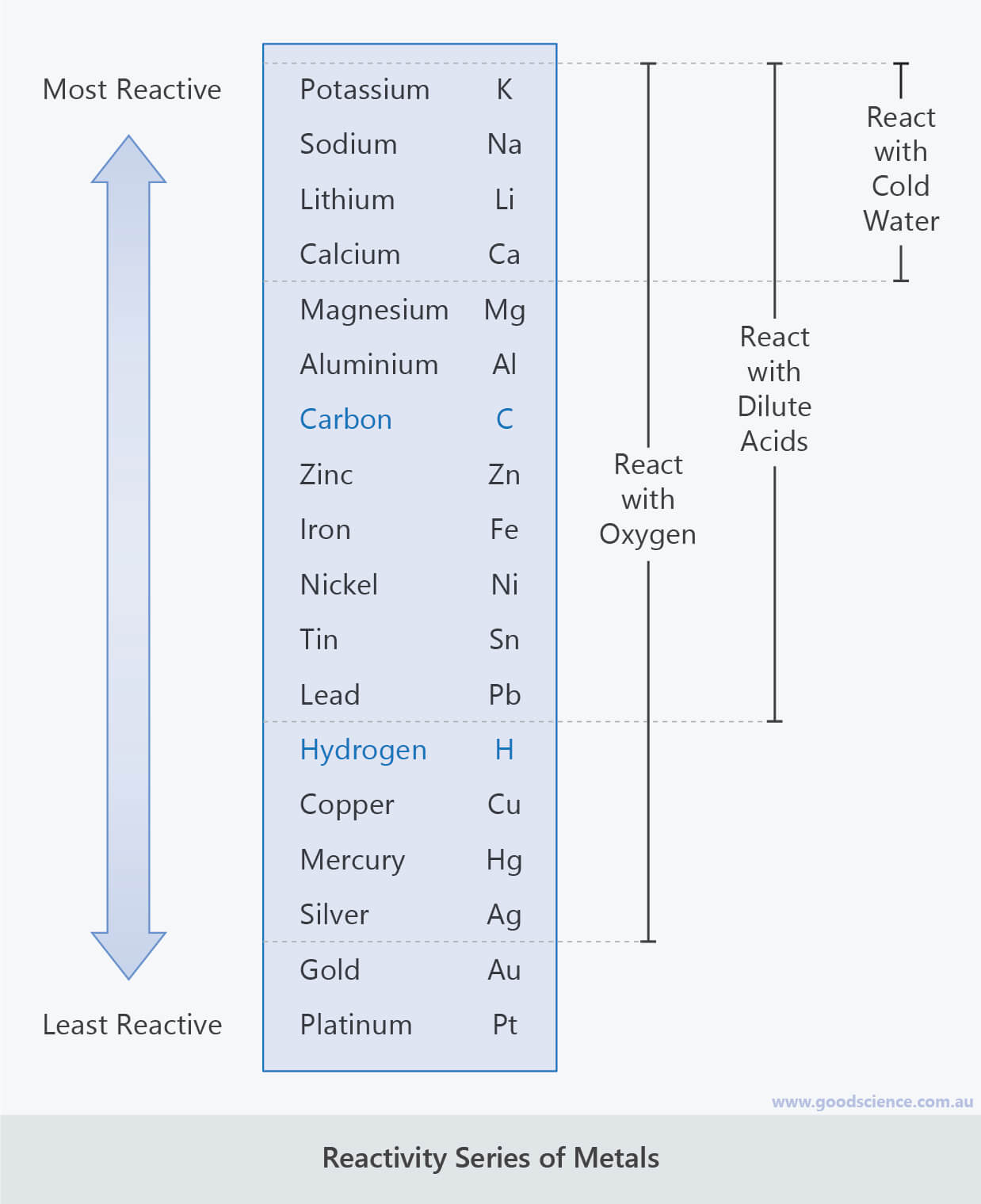

There is some ambiguity at the borderlines between the groups. Metals in the middle of the reactivity series, such as iron, will react with acids such as sulfuric acid (but not water at normal temperatures) to give hydrogen and a metal salt, such as iron(II) sulfate:įe (s) + H 2SO 4 (l) → FeSO 4 (aq) + H 2 (g) The most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide:Ģ Na (s) + 2 H 2O (l) →2 NaOH (aq) + H 2 (g) There is no unique and fully consistent way to define the reactivity series, but it is common to use the three types of reaction listed below, many of which can be performed in a high-school laboratory (at least as demonstrations). become stronger reducing agents ( electron donors).require more energy (and different methods) to be isolated from their compounds.lose electrons ( oxidize) more readily to form positive ions.Going from the bottom to the top of the table the metals: May react with some strong oxidizing acids Reacts with acids very poor reaction with steam Or less commonly other alkali metals, hydrogen or calcium in the Kroll process Pyrometallurgical extraction using magnesium, In boiling water, and very vigorously with acids Reacts very slowly with cold water, but rapidly 3 Comparison with standard electrode potentialsĮlectrolysis (a.k.a.


 0 kommentar(er)
0 kommentar(er)
